Do you search for 'thesis steam reforming'? You can find all the material on this web page.
Table of contents
- Thesis steam reforming in 2021
- Steam reforming method
- Thesis steam reforming 03
- Thesis steam reforming 04
- Thesis steam reforming 05
- Thesis steam reforming 06
- Thesis steam reforming 07
- Thesis steam reforming 08
Thesis steam reforming in 2021
 This image representes thesis steam reforming.
This image representes thesis steam reforming.
Steam reforming method
 This image demonstrates Steam reforming method.
This image demonstrates Steam reforming method.
Thesis steam reforming 03
 This image shows Thesis steam reforming 03.
This image shows Thesis steam reforming 03.
Thesis steam reforming 04
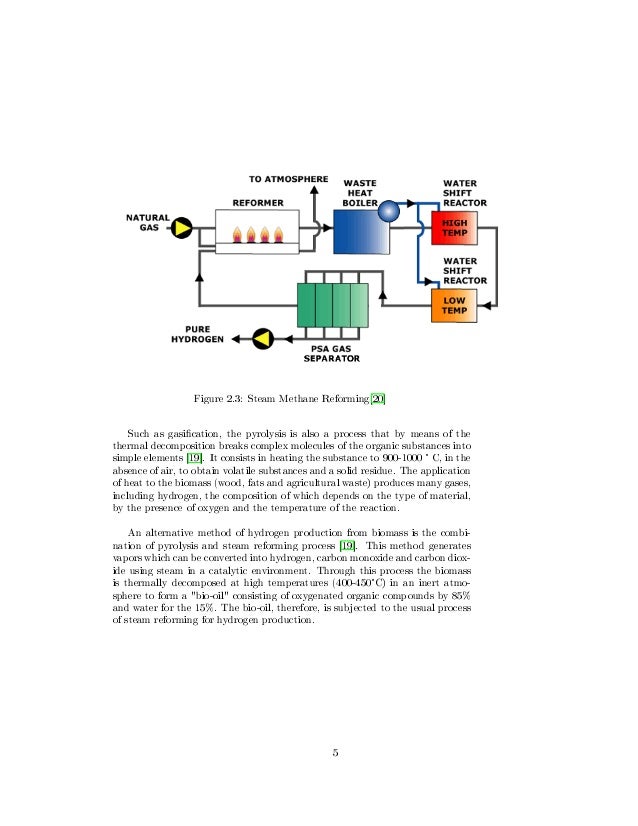 This image illustrates Thesis steam reforming 04.
This image illustrates Thesis steam reforming 04.
Thesis steam reforming 05
 This picture shows Thesis steam reforming 05.
This picture shows Thesis steam reforming 05.
Thesis steam reforming 06
 This picture shows Thesis steam reforming 06.
This picture shows Thesis steam reforming 06.
Thesis steam reforming 07
 This picture demonstrates Thesis steam reforming 07.
This picture demonstrates Thesis steam reforming 07.
Thesis steam reforming 08
 This image representes Thesis steam reforming 08.
This image representes Thesis steam reforming 08.
Which is the first reaction in methane reforming?
Here, the first reaction CH4+ H2O = CO + 3H2, i.e., methane reforming, is analyzed using a reaction route network approach to obtain the overall methane steam reforming network and kinetics. Kinetics providing detailed information of elementary reaction steps for this system, namely micro-kinetics, has not yet been fully addressed.
What is the efficiency of steam methane reforming?
Steam methane reforming is 65-75% efficient, so a percentage of methane remains unreacted after the process is complete. Carbon dioxide is often captured and used for enhanced oil recovery in the refining industry, while the other leftover gases can be used as fuel for the furnace. The remaining hydrogen is now ready for the customer.
How is hydrogen produced in the steam reforming process?
Steam Methane Reforming Process First, water must be heated in a furnace to produce steam. The superheated steam is mixed with natural gas in the reforming reaction, producing hydrogen gas and carbon monoxide. Carbon monoxide from the reforming reaction interacts with water again to produce more hydrogen and carbon dioxide.
What is the ratio of steam to carbon in steam reforming?
In addition to computing the micro-kinetic model, experimental work for methane steam reforming was conducted. A steam to carbon ratio of 2:1 was fed to the packed bed reactor, where experimental conversion data were obtained.
Last Update: Oct 2021